blr_p
Quasar
Using citric acid to descale a kettle. This video shows it works. He even used it with a coffeemaker
What prompted this post? Karcher does not like this idea
Similar things are said about not using citric acid to descale coffeemakers as well. Don't use citric acid as at high temperatures, bla bla. How high?
 chemistry.stackexchange.com
chemistry.stackexchange.com
Of course, they don't want you using a home remedy when they have a product to sell. Which costs a bit and what better way to make you buy their product than to scare the crap out of you from using alternatives
Are there better chemicals than citric acid? Yeah, Sulfamic acid for one. Some of these descalers have it. Works better than citric acid to descale and is used to descale coffeemakers.
The trouble with sulfamic acid is it can take the shine off your steel drum, over time. It depends on the steel. Some grades are fine, others are not. That person complaining discovered it did not work very well with the steel used with her Bosch machine. But I've seen similar complaints with other machines so yeah don't descale your washing machine with a coffee machine descaler.
What prompted this post? Karcher does not like this idea
They prefer you use lower temperatures to descale. But where is the hard-to-remove calcium citrate in the kettle video?Using citric acid as an alternative
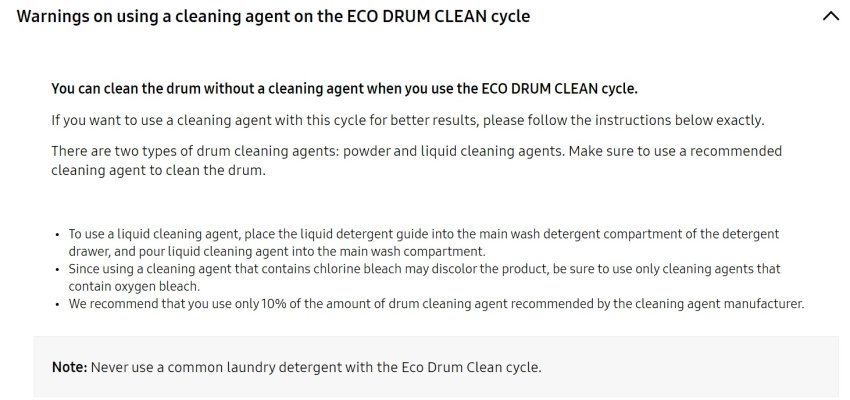
In principle, a dishwasher can also be descaled with citric acid, but there a few things to keep in mind here. At high temperatures, citric acid combines with lime to form calcium citrate, which is very difficult to remove. To descale, add approximately 10 tablespoons of citric acid to 2 litres of cold water. Start the unfilled dishwasher on the lowest temperature possible. As soon as water has been distributed in the dishwasher, stop the programme and pour in the water-citric acid mix. Close the dishwasher and leave for 1 hour, then start the programme again. The advantage over vinegar or vinegar essence is the pleasant citrus fragrance.
Similar things are said about not using citric acid to descale coffeemakers as well. Don't use citric acid as at high temperatures, bla bla. How high?
Limescale passivation by citric acid?
Cleaning products producers advise against using "home remedies" instead of their products. This advice against using citric acid to remove limescale was especially surprising for me: Ci...
There is no cure-all solution for descaling and no "reaction" because scale is not a standard compound. Its composition will vary with water quality. So it is a bad idea by these companies to spread rumors that citric acid passivates limescale.
One has to know what the scale is and the construction material, to begin with descaling. I think it all depends on the application. For example, citric acid is ideal for descaling dishwashers and washing machines. It is not corrosive to steel and forms complexes with iron, if there are rust stains. Note that descaling is done with citric acid (citric acid is in excess) in these machines.
Of course, they don't want you using a home remedy when they have a product to sell. Which costs a bit and what better way to make you buy their product than to scare the crap out of you from using alternatives

Are there better chemicals than citric acid? Yeah, Sulfamic acid for one. Some of these descalers have it. Works better than citric acid to descale and is used to descale coffeemakers.
The trouble with sulfamic acid is it can take the shine off your steel drum, over time. It depends on the steel. Some grades are fine, others are not. That person complaining discovered it did not work very well with the steel used with her Bosch machine. But I've seen similar complaints with other machines so yeah don't descale your washing machine with a coffee machine descaler.
Last edited:

 That is why you need to test acidity
That is why you need to test acidity